Vivosun Digital pH Meter Set: Everything You Need to Measure and Adjust pH Levels
Table of Contents
Discussing the necessary preparations before conducting pH measurements
Preparing for pH measurements is an essential step in ensuring accurate and reliable results. One of the first things to consider is the calibration of the pH meter. This process involves checking the accuracy of the device by comparing it to standard pH solutions. By calibrating the meter before each measurement, you can minimize any potential errors caused by instrument drift or deviation.
Another crucial aspect of preparation is sample collection and handling. It is important to collect a representative sample that accurately reflects the pH of the solution or substance you wish to measure. Avoiding cross-contamination is also vital, as even small traces of other substances can alter the pH reading. Clean and sterilize any equipment used in sample collection to minimize any interference.
Additionally, factors such as temperature can have an impact on pH measurements. It is crucial to take note of the temperature of the sample and adjust the pH reading accordingly, as pH values can vary depending on temperature. Many pH meters have built-in temperature compensation features to aid in accurate measurements.
In conclusion, thorough preparation is crucial before conducting pH measurements. Calibration of the pH meter, careful sample collection, and temperature considerations all play a role in obtaining accurate and reliable results. By following these necessary steps, you can ensure that your pH measurements are precise and useful in various applications, from gardening to scientific research.
– Calibrate the pH meter before each measurement to ensure accuracy
– Collect a representative sample that accurately reflects the pH of the solution or substance being measured
– Avoid cross-contamination by cleaning and sterilizing equipment used in sample collection
– Take note of the temperature of the sample and adjust pH readings accordingly, as pH values can vary with temperature
– Use pH meters with built-in temperature compensation features for more accurate measurements
Exploring the importance of sample
When it comes to conducting pH measurements, one of the most crucial aspects to consider is the sample itself. The quality and composition of the sample can have a significant impact on the accuracy and reliability of the pH measurements obtained.
First and foremost, it is important to ensure that the sample is representative of the system or environment being analyzed. This means that the sample should be taken from a homogeneous and well-mixed portion of the system, avoiding any areas that may introduce biased results. Additionally, proper care should be taken to prevent any external contaminants from coming into contact with the sample, as even slight contamination can alter the pH readings.
Furthermore, the sample size also plays a role in obtaining accurate pH measurements. Ideally, the sample should be of sufficient volume to allow for multiple measurements to be taken, ensuring repeatability and reducing the possibility of random errors. Additionally, for samples with varying pH levels or gradients within the system, it may be necessary to take multiple samples at different locations to obtain a comprehensive understanding of the pH distribution.
In summary, the importance of sample quality and preparation cannot be overstated when it comes to conducting pH measurements. By ensuring that the sample is representative, free from contamination, and of appropriate size, we can improve the accuracy and reliability of our pH measurements, ultimately leading to more informed decisions and successful outcomes in various applications.
• The quality and composition of the sample can impact the accuracy and reliability of pH measurements.
• The sample should be representative of the system or environment being analyzed.
• Care should be taken to prevent external contaminants from coming into contact with the sample.
• Sample size is important for obtaining accurate pH measurements.
• Sufficient volume allows for multiple measurements, ensuring repeatability and reducing random errors.
• Multiple samples may be necessary for systems with varying pH levels or gradients.
What are the necessary preparations before conducting pH measurements?
Before conducting pH measurements, it is important to properly calibrate the pH meter using standard buffer solutions. Additionally, ensure that the pH electrode is clean and free from any residue or contaminants. Properly prepare the sample solution by ensuring it is well-mixed and at the appropriate temperature.
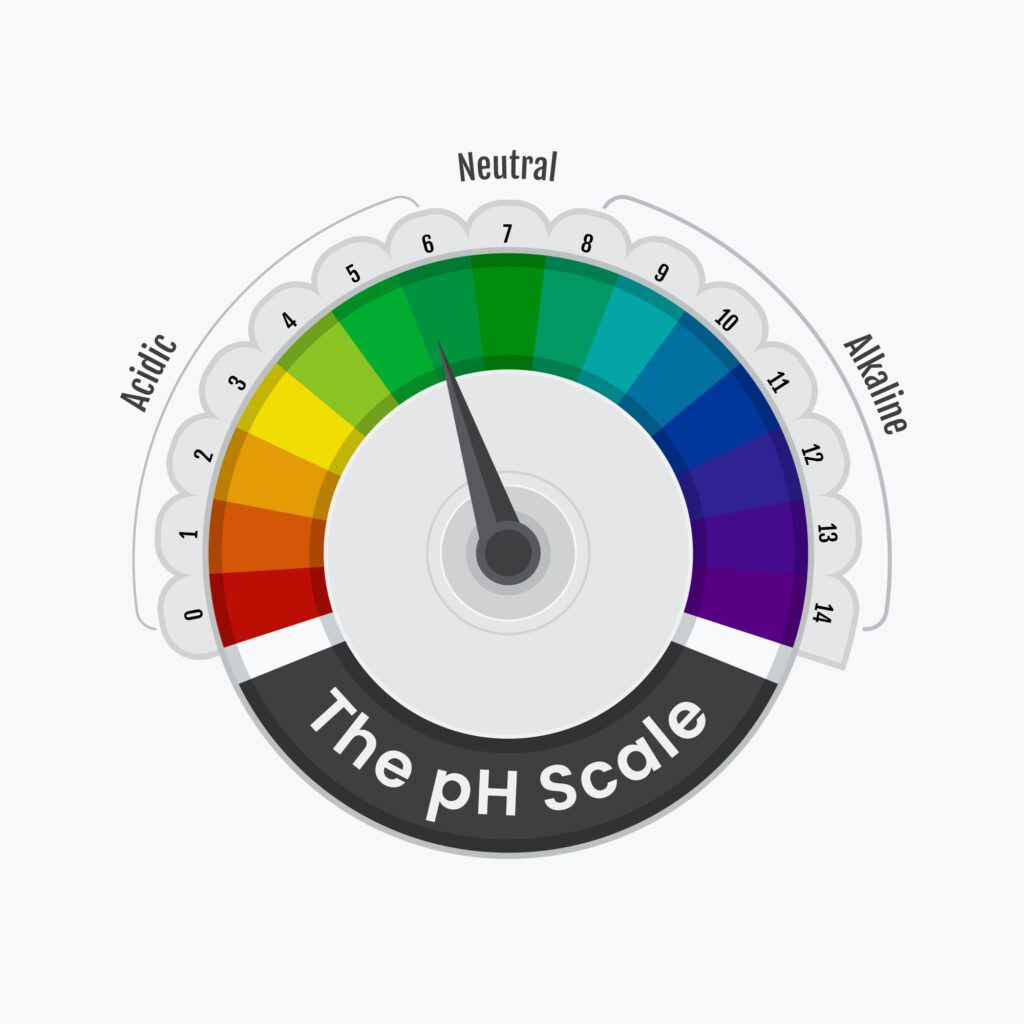
Why is it important to calibrate the pH meter before conducting measurements?
Calibrating the pH meter using standard buffer solutions ensures the accuracy and reliability of the pH measurements. It allows for proper calibration of the electrode and adjustment for any potential measurement errors, resulting in more precise and meaningful data.
How should the pH electrode be cleaned before measurements?
The pH electrode should be cleaned by rinsing it with distilled water to remove any residue or contaminants. It can then be soaked in a cleaning solution specifically designed for pH electrodes or a mild detergent solution. Gently scrub the electrode using a soft brush if necessary, and rinse it thoroughly with distilled water before use.
Why is it important to properly prepare the sample solution?
Properly preparing the sample solution ensures that it is homogeneous and representative of the system being studied. It helps to eliminate any potential sources of error or bias in the pH measurements. Additionally, ensuring the sample solution is at the appropriate temperature allows for more accurate and consistent results.
Can I use any buffer solutions for pH meter calibration?
It is recommended to use certified buffer solutions with known pH values for pH meter calibration. These buffer solutions are specifically designed to provide accurate and reliable calibration points. Using uncertified or homemade buffer solutions may introduce errors and result in inaccurate pH measurements.

How often should I calibrate the pH meter?
The frequency of pH meter calibration depends on various factors such as the stability of the pH meter, frequency of use, and the importance of accuracy in the measurements. However, a general guideline is to calibrate the pH meter at the beginning of each day or before starting a new set of measurements. Regular calibration helps to maintain the accuracy of the pH meter over time.
Can I reuse the sample solution for multiple pH measurements?
It is generally not recommended to reuse the sample solution for multiple pH measurements. Each measurement may slightly alter the composition of the sample, affecting its pH value. To obtain accurate and reliable results, it is best to prepare fresh sample solutions for each measurement.
How long should I wait for stable pH readings after immersing the electrode in the sample solution?
The time required for stable pH readings can vary depending on the nature of the sample solution and the pH meter being used. However, it is generally recommended to wait for at least 30 seconds to 1 minute for the pH readings to stabilize. This allows the electrode to equilibrate with the sample and provide more accurate measurements.
Can the pH meter be used for measuring the pH of any type of sample?
The pH meter can be used for measuring the pH of a wide range of samples, including liquids, semi-solids, and even some solids. However, it is important to select the appropriate type of pH electrode and sample preparation method based on the nature and characteristics of the sample being tested.
To learn more about the Vivosun Digital pH Meter watch the video below:

Appreciate it for all your efforts that you have put in this. very interesting info .